Pharmacology and Drug Discovery
Overview Service
BRF is a leading Contract Research Organization (CRO) that offers comprehensive pharmacology and drug discovery services, catering to the needs of the pharmaceutical and biotechnology industries. With our expertise, state-of-the-art facilities, and dedicated team of scientists, we are committed to supporting our clients in advancing their drug discovery programs and accelerating the development of life-saving therapies.
Our pharmacology and drug discovery services encompass a wide range of capabilities, designed to address the complex challenges faced by the industry. We offer preclinical studies, in vitro and in vivo pharmacology assays, efficacy and safety evaluations, and specialized testing across various therapeutic areas.
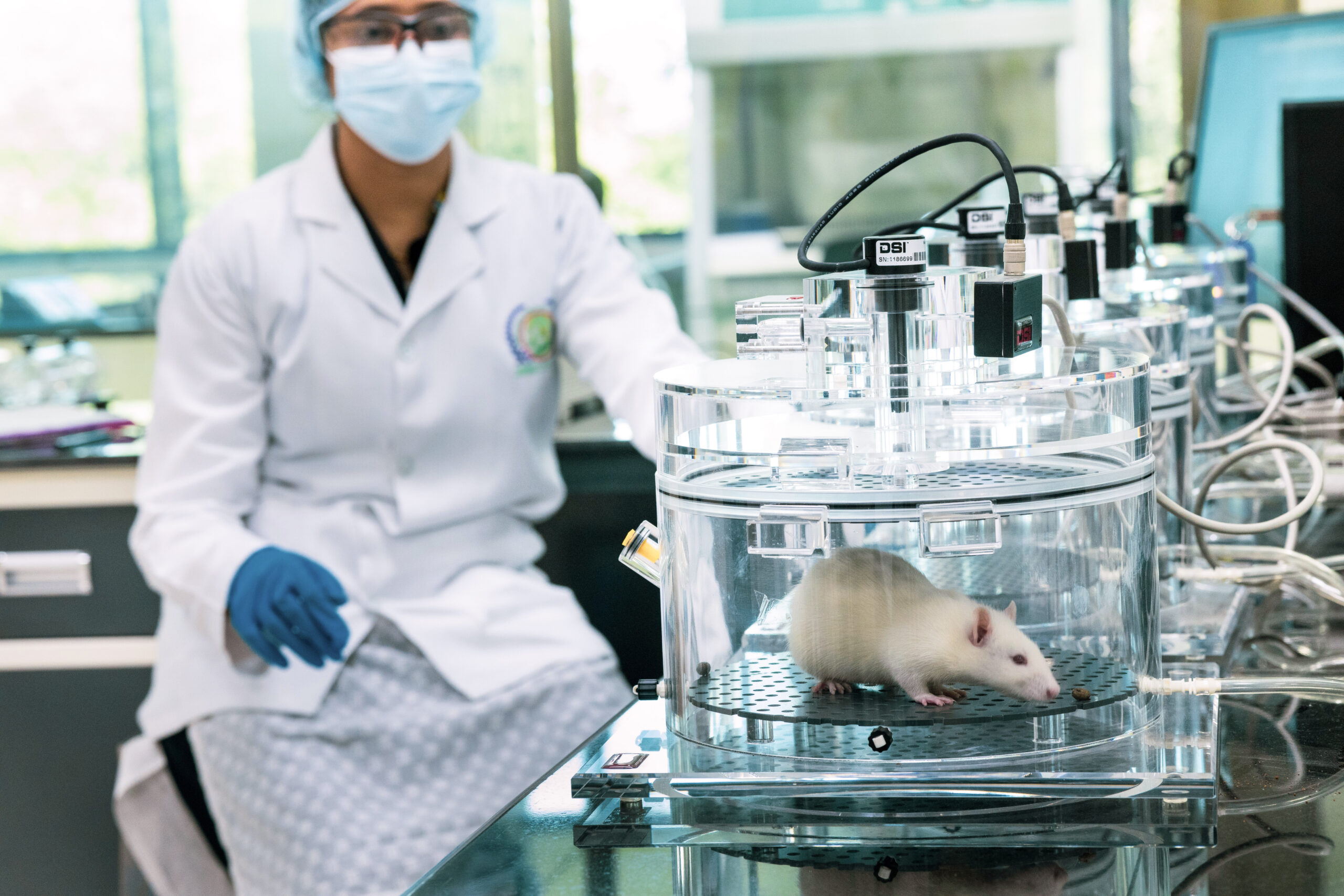
At BRF, we understand the importance of early-stage drug discovery and the need for reliable and accurate data. Our experienced scientists leverage their expertise in designing and executing preclinical studies to assess the safety, efficacy, and pharmacokinetic properties of novel drug candidates. We employ a variety of assays and models (Small to Large animal models like canine, Swine, Livestock etc) to evaluate drug activity, ADME (Absorption, Distribution, Metabolism, and Excretion) properties, toxicity profiles, and target engagement.
In our state-of-the-art laboratories, we utilize advanced technologies and cutting-edge equipment to conduct comprehensive pharmacological evaluations. Our facilities are equipped with a wide range of in vitro and in vivo screening platforms, including high-throughput screening (HTS), cell-based assays, animal models, and imaging techniques. These capabilities enable us to generate robust and reliable data, providing valuable insights into the potential of drug candidates.
Furthermore, BRF offers specialized services in areas such as pharmacokinetics, toxicology, safety pharmacology, biomarker analysis, and drug metabolism studies. We employ advanced analytical techniques to assess drug metabolism, identify biomarkers, and evaluate potential drug-drug interactions. Our expertise in these areas allows us to provide comprehensive support throughout the drug discovery and development process.
Collaboration is at the core of our approach. We work closely with our clients to understand their specific needs and goals, tailoring our services to meet their unique requirements. Our team of skilled scientists and project managers ensures efficient project execution, timely delivery of results, and open communication at every stage of the engagement.
As a trusted partner in the pharmacology and drug discovery service industry, BRF is dedicated to delivering high-quality data, scientific excellence, and regulatory compliance. Our commitment to Good Laboratory Practices (GLP) and adherence to industry standards ensure the integrity and reliability of our results.
Partnering with BRF provides pharmaceutical and biotechnology companies with a competitive advantage, allowing them to make informed decisions, optimize their drug discovery strategies, and accelerate the development of novel therapies. By leveraging our expertise, cutting-edge technologies, and commitment to scientific rigor, clients can confidently advance their drug candidates through the discovery pipeline.
In summary, BRF is a leading CRO that offers comprehensive pharmacology and drug discovery services to support the pharmaceutical and biotechnology industries. Our expertise, state-of-the-art facilities, and dedicated team empower our clients to make informed decisions and accelerate the development of life-changing therapies. Partner with BRF and unlock the potential of your drug discovery programs.
Our Lab


Overview of services
- Absorption, Distribution, Metabolism, and Excretion (ADME) profiling
- Plasma protein binding
- Metabolite identification and characterization
- Bioavailability and bioequivalence studies
- Drug-drug interaction studies
- Pharmacokinetic modeling and simulation
- Receptor binding assays
- Enzyme inhibition assays
- Cell-based assays
- In vitro ADME assays
- Animal models for efficacy testing
- Pharmacological profiling of compounds
- Safety pharmacology assessment
- Central nervous system (CNS) safety studies
- Respiratory system safety studies
- Gastrointestinal system safety studies
- Renal and hepatic safety studies
- Integrated risk assessment
- Drug metabolism and pharmacokinetic profiling
- Cytochrome P450 inhibition and induction assays
- Metabolite profiling and identification
- Tissue distribution studies
- Bioavailability and bioequivalence testing
- Pharmacokinetic modeling and simulation
- Drug-drug interaction studies
- Transporter interaction studies
- Drug-drug interaction potential assessment
- Evaluation of drug metabolism pathways
- In vitro and in vivo interaction studies
- Clinical drug interaction studies
- Pharmacodynamics and efficacy testing
- Biomarker analysis
- Cellular signaling pathway analysis
- In vivo efficacy studies
- Dose-response relationship studies
- Clinical trial support
