Safety Pharmacology
Overview Service
Safety Pharmacology is a crucial aspect of drug development that evaluates the safety of new drugs before they are administered to humans. At our Safety Pharmacology division, we are committed to providing comprehensive safety evaluation of new drugs through cutting-edge research and state-of-the-art technology.
Our laboratory is equipped with the latest equipment and facilities necessary to conduct safety pharmacology studies. Our reports are accepted worldwide and conform to the pharmacopeia of various regulated markets, ensuring that our clients’ drugs meet the necessary regulatory requirements for distribution.
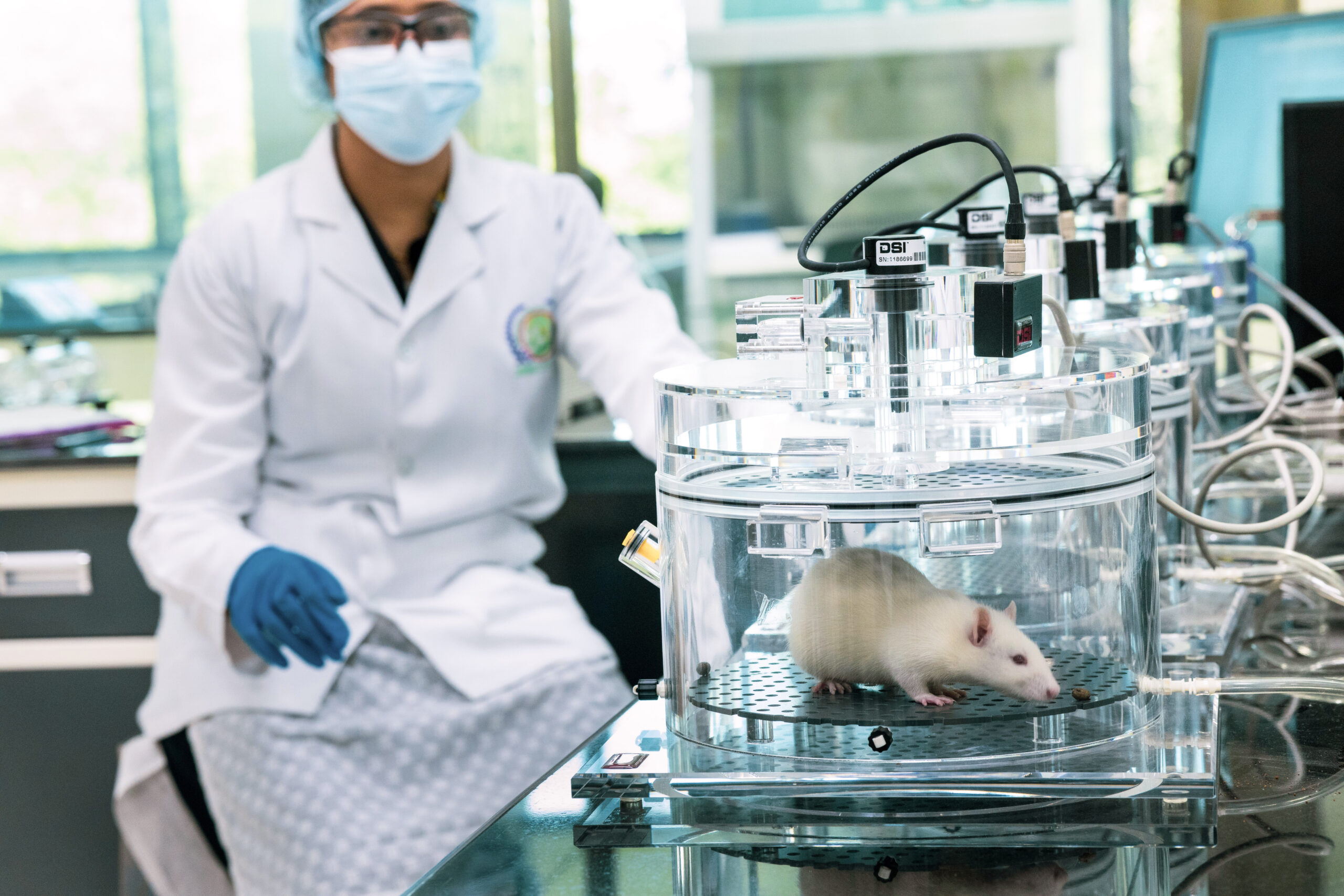
We have a dedicated AAALAC-affiliated small and large animal house for small and large animal tests, including DMPK/PD, Telemetry, Oncology, and range-finding studies. Our in-house full-time board-certified pathologists and veterinarians ensure the well-being of the animals and the accuracy of the studies.
At our Safety Pharmacology division, we provide a comprehensive range of safety pharmacology services, including cardiovascular and respiratory safety assessment, CNS safety assessment, and hERG screening. Our experienced team of scientists works closely with clients to design and conduct studies that meet their specific needs.
Other services include high-throughput screening, in vitro ADME (Absorption, Distribution, Metabolism, and Excretion) studies, in vivo pharmacokinetic and pharmacodynamic studies, safety pharmacology, and regulatory toxicology. We also offer bioanalytical services, such as method development, validation, and sample analysis.
Our mission is to ensure the safety of new drugs before they are administered to humans, ultimately reducing the risk of adverse drug reactions and improving patient safety. We invite you to explore our website to learn more about our research, facilities, and expertise. Please feel free to contact us for any safety pharmacology studies you may require.
Our Lab


Quick overview of services we can provide
- Evaluation of the effects of drug candidates on cardiovascular function.
- Telemetry studies for monitoring cardiovascular parameters in conscious animals
- ECG (Electrocardiogram) assessment
- Blood pressure monitoring
- Assessment of the effects of drug candidates on the central nervous system.
- Functional observational battery (FOB)
- Neurobehavioral assessments
- Cognitive function testing
- Evaluation of the effects of drug candidates on respiratory function.
- Pulmonary function
- Respiratory rate and pattern assessments
- Bronchoconstriction studies
- Assessment of the effects of drug candidates on renal and urinary function.
- Urine volume and composition analysis
- Renal clearance studies
- Kidney histopathology evaluation
- Evaluation of the effects of drug candidates on gastrointestinal function.
- Gastric emptying studies
- GI motility assessments
- Intestinal permeability studies
- Assessment of the effects of drug candidates on hepatic and metabolic function.
- Liver function
- Enzyme induction/inhibition studies
- Metabolic profiling
- Evaluation of the effects of drug candidates on ocular function and structure.
- Ophthalmic examinations
- Intraocular pressure measurements
- Ocular histopathology evaluation
- Assessment of the effects of drug candidates on the immune system.
- Immune cell profiling
- Cytokine analysis
- Immunophenotyping
- Evaluation of the effects of drug candidates on reproductive and developmental function.
- Fertility and reproductive performance studies
- Embryo-fetal development studies
- Teratology studies
- Evaluation of the combined effects of drug candidates on different physiological systems.
- Combination cardiovascular and CNS assessments
- Combination respiratory and GI system assessments
- Combination renal and hepatic system assessments
